Sunday, August 9, 2009
The Periodic table
• The lements are arranged in periods and groups.
Period:
• A period is a horizontal raw of elements.
• There are seven periods.
• The period number refers to the number of shells present in an element.
For example: The element carbon and sodium.
Carbon has two shells, therefore it belongs to period two. Like wise sodium has three shells so it belongs to period three.
• First period consists of two elements. They are hydrogen and Helium.
• The second and the third period consists of eight elements.
• The fourth and the fifth period consists of eighteen elements.
Friday, June 5, 2009
Giant Molecular structure
E.g.: - Diamond, graphite, silicon (IV) oxide (silica)
• Each single crystal if a diamond is one giant molecule. carbon atom is bonded to other carbon atoms tetrahedrally.
• All the four outermost electrons in carbon atom are involved in the bond formation and there are no free electrons to move. therefore diamond does not conduct electricity.
• A lot if energy is required to break apart the strong covalent bonds between the carbon atoms, hence diamond has very high melting & boiling point.
• Diamond is the hardest substance known since the carbon atoms are not able to slide over each other due to strong covalent bonds.
• Diamond is a transparent, colourless crystal.
Structure of Graphite
• Graphite has a layered structure each carbon is bonded to three other carbon atoms in a hexagonal arrangement to form rings of regular hexagons. the different layers are held together by weak vanderwaal's force of attraction.
• Graphite has very high melting & boiling point. therefore a lot of energy is required to break apart the strong covalent bonds between the carbon atoms.
• It is a good conductor of electricity because each carbon atom uses only 3 out of 4 outer electrons for bonding with one free electron to conduct electricity.
• The weak vanderwaal's force between the layers enables layers to slide over each other. hence graphite is a soft substance.
• It is black, opaque and shiny solid.

Structure of Polythene
• Polythene is a plymer. It is made up of thousands of molecules called monomers joined together in long chains. The monomer used in making polythene is called ethene.
• Polythene has high melting point and does not conduct electricity.
• Polythene can be softened on heating and melting and set again when cooled.
Monday, June 1, 2009
Simple Molecular structure
• A simple molecular structure contains small molecules.
• Simple molecular structure are formed from only a few atoms.
• They have strong covalent bonds between the atoms with in a molecule, what have weak bonds between molecules is called as Vanderwaal’s force.
• These vanderwaal’s forces increase steadily with the increasing size of the molecules.
• Most of the simple molecular structures are liquids or gases under normal conditions.
• In simple molecular structures the forces between the molecules in the solid and liquid state are weak such that very little energy is needed to break up the structure.
E.g.: - Methane (CH4), Iodine (I2), Water (H2O), Carbon dioxide (CO2)
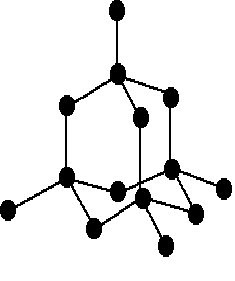
Structure of Methane
This is the tetrahedral shake of methane molecule. The carbon-hydrogen bond is a strong covalent bond. There is weak vanderwaal’s force is between the molecules of methane.
Structure of Iodine
Iodine has a crystal structure in which iodine molecules are packed together. The force between the molecules are weak so iodine is a flaky solid that sublimes if heated gently iodine crystals can be crushed easily.
Elements, Compounds & Mixtures
• An element is made up of only one types of atom.
• Elements are substances that cannot be broken down chemically into smaller substances.
E.g.: - Carbon, Nitrogen, Oxygen, Calcium, Helium etc.
Compounds:
• A compound is made up of two or more different kinds of atoms chemically combined together.
E.g.: - Water, Hydrochloric acid, Carbon monoxide etc.
Mixtures:
Mixtures are the substances that are simply mixed together without any chemical reaction taking place between them. A mixture is made up of two or more elements or compounds physically combined together. The individual substances can be separated by physical means.
E.g.: - Air, Sea water etc.
Tuesday, May 26, 2009
Electronic configuration of Ions
• Ions with single positive charge are formed when an atom loose one electron.
E.g.: - Sodium atom (Na) loose one electron to form sodium ion (Na+)
E.g.: - Beryllium atoms (Be) loose 2 electrons to form beryllium ion (Be²).
• Negative ions are formed when atoms gain electrons.
• Ions with single negative charge are formed when an atom gains one electrons
E.g.: - Chlorine (Cl) atom gains one electron to form chloride ion (Cl−).
E.g.: - Oxygen (O) atom gain 2 electrons to form oxide ion (O²−).
•
Friday, May 22, 2009
Electron Arrangement in Atoms
• This arrangement of electrons around the nucleus of an atom is called electronic configuration.
• Shells are numbered starting from the shell which is nearest to the nucleus of the atom.
• Hence the shell which is furthest away from nucleus is called the outermost shell and the electrons in the shell are referred to as the outermost electrons.
• The shell nearest to nucleus is called the first shell and this is also the lowest energy level.
• Each shell can hold a fixed number of electrons. The formula helps us to find the number of electrons in each shell. And the formula is 2n² where ‘n’ is the shell number.
For the first 20 elements (hydrogen to calcium)
» The first shell (lowest energy level) can hold maximum of 2 electrons.
» The second shell can hold a maximum of 8 electrons.
» The third shell can hold a maximum of 8 electrons.
» The remaining electrons are placed in the fourth shell.
» The energy levels must be filled in order of increasing energy. The first level is filled first before going to the second level and subsequent higher levels.

Radioactive Isotopes
• Isotopes of some elements are radioactive.
• Radioactive isotopes have unstable nuclei which breakup and emit radiations.
• Nucleus if these isotopes contain extra number of neutrons than in the number of protons.
| Isotopes of certain elements with unstable nuclei which breakup to give off one or more types of radiation are known as radioactive isotopes. |
→ Radioactive isotopes can emit:
• Alpha particles
• Beta particles
• Gamma rays
E.g. of radioactive isotopes: hydrogen-3(Tritium), carbon-14, uranium-235
Thursday, May 21, 2009
Isotopes
• Atoms of an element are not always identical.
• All the atoms of the same element can contain different number of neutrons.
• These atoms are called Isotopes.
• Isotopes are atoms of the same element having same atomic number but different mass number.
E.g.: - Hydrogen has 3 isotopes.

• Isotopes of the same element have similar decimal properties but different physical properties.
E.g.: - Isotopes if hydrogen that is Protium, Deuterium and Tritium have different boiling point.
• This is because the chemical property of an atom depends on the number of protons & electrons of an atom as isotopes of the same element have same atomic number (proton number) they have similar chemical properties.
Wednesday, May 20, 2009
Atomic Structure
| Particles | Relative mass | Charge | Location in the atom |
| Protons (p) | 1 | +1 (positive) | In the nucleus |
| Neutrons (n) | 1 | 0 (zero) | In the nucleus |
| | 1/1840 (or) 0.0005 | –1 (negative) | Arranged in energy levels or shells around the nucleus |
• The nucleus is at the center of the atom and contains the protons and neutrons.
• The protons and neutrons are collectively known as nucleons and they are larger and heavier than electrons.
• Electrons orbit around the nucleus in shells or energy levels like planets revolving around the sun.
• Protons carry up positive charge (+), neutrons have no charge therefore it is neutral and electrons have a negative charge (−).
• Atoms are neutral.
• In a neutral atom the number of protons (+) equals to the number of electrons (−) that is the number of positive charges is equal to the number of negative charges and the charges cancel each other out.
• The structure of an atom can be represented in the symbol form:

Atomic number (Z):
• Atomic number (Z) is the number of protons in the nucleus of an atom.
• It is also known as the proton number of the particular element.
| ATOMIC NUMBER = number of protons |
• Each element has it's own symbol and own kind of atoms with fixed number of protons.
• As the proton number for each element is fixed it can be used to identify atoms of different elements.
E.g.: - The atomic number (proton number) for:
• Hydrogen is 1, therefore it is written as H.
• Carbon is 6, therefore it is written as C.
Mass number (A):
• Mass number (A) is the sum of the number of protons and the number of neutrons in one atom of an element.
| MASS NUMBER = number of protons + number of neutrons |
• Mass of an atom includes mass of protons and neutrons present in that particular atom. As protons and neutrons are present in the nucleus of an atom, it is also known as Nucleon number.
• Electrons are too small to affect the mass of an atom.
Structure of atoms in symbol form
• Atomic number, mass number and the symbol f
or an element can be used ti write the structure of an atom in the symbol form. (As it appears in the periodic table)
E.g.: - Atomic number of the calcium atom is 20 and mass number is 40.
• Therefore, the structure if the atom of calcium can be represented as:
Neutron number (n): is the number of neutrons present in an atom. It can be found using the formula.
| NEUTRON NUMBER = mass number − atomic number |
• Therefore, for calcium atom
IN SUMMARY:
Atomic number = Number of protons.
Mass number = Number of protons + number of neutrons.
Neutrons number = Mass number − atomic number